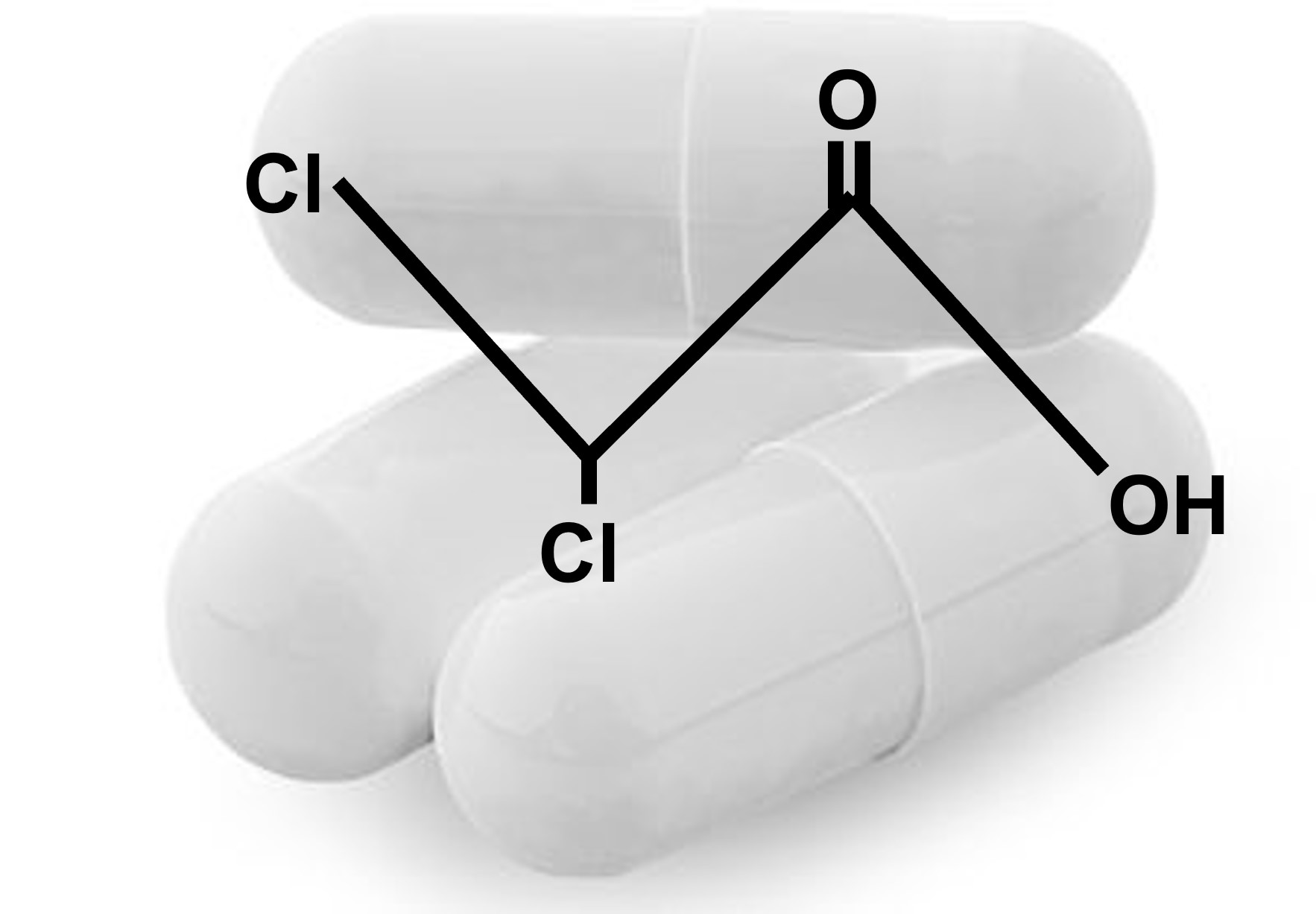
Description
Dichloroacetate (DCA) is a substance that is being used for medical treatment of rare congenital forms of lactic acidosis, particularly pyruvate dehydrogenase complex (PDC) deficiency. The current interest in DCA as a cancer remedy evolved after the publication of a scientific article in 2007 that reported the ability of this compound to cause selective death of human cancer cells studied in tissue culture or after implantation into animal hosts. Subsequent claims by various non-scientific, for-profit groups were directed at the lay public regarding DCA’s purported anti-cancer effectiveness, based on results from the original pre-clinical studies and subsequent mostly unverifiable testimonials.
Efficacy
Following the initial report, several independent researchers have confirmed and extended the original findings of DCA’s anti-neoplastic activity in a variety of human cancer cells and animal models. These studies provide evidence that DCA interacts with fundamental metabolic and signalling pathways to inhibit malignant cell proliferation and could increase the effect of several anti-cancer substances.
No controlled clinical trials of DCA are available. The results of four small phase I trials, in which safety was the primary outcome, suggest that chronic administration of DCA as a single substance is generally well tolerated and might have effects against some human tumours.
Safety
DCA is generally well tolerated; doses up to 6.25 mg/kg body weight twice daily can be administered safely to most subjects.
Description
Dichloroacetate (DCA) is a xenobiotic, meaning that it does not occur naturally in our bodies or in the environment. However, DCA is widely present in the environment in minute quantities because it is a by-product of the process of water chlorination. It is also a product of the breakdown of certain industrial chemicals and medicinal drugs. DCA is also used as an intermediate for chemical syntheses1. The U.S. Environmental Protection Agency (EPA) has classified DCA as a hazardous environmental chemical (group B2, probable human carcinogen)2. On the other hand, it is being used as an investigational drug for the treatment of cancer3 and of rare diseases associated with disturbances of cellular energy metabolism, such as congenital lactic acidosis4,5.
Scientific name/ingredients
DCA mostly refers to the sodium or potassium salts of dichloroacetic acid. DCA salts can be easily solved in water. Pure dichloroacetic acid is a strong organic acid and should not be ingested.
Application and dosage
DCA has been administered to patients with genetic mitochondrial diseases for more than 30 years and its pharmacology has been extensively studied6,7. DCA is rapidly absorbed after oral intake, has a plasma half-life time of approximately 1 hour and inhibits its own metabolic breakdown leading to increasing plasma concentrations over time8. There is evidence that the clearance of DCA and possibly neurotoxic degradation products correlates with age and depends on the subject’s genotype of the glutathione transferase zeta-19,10. The doses of DCA applied orally in clinical studies range between 4 mg/kg body weight twice daily to 25mg mg/kg body weight twice daily. A dosage of 6.25 mg/kg body weight twice daily leads to plasma concentrations at values required for the inhibition of pyruvate dehydrogenase kinase11.
In addition, variations in the rate at which DCA is metabolized in humans suggest that genetics-based personalized dosing of DCA may offer an improved means of administering safe doses12.
History/providers
DCA made its way into the press and media due to results that were derived from laboratory and animal studies13.
The principal commercial supplier of DCA for laboratory and clinical research subsequently marketed DCA as being “the best new approach to cancer treatment in years”) via its Website. The Food and Drug Administration (FDA) banned the website in 2007 and shut down sales of DCA.
Claims of efficacy
The DCA story and the associated claims raised hope for a cancer cure and received widespread attention. Therefore, DCA is often being labeled as a wonder drug against cancer in the lay media, whereas other critical voices described it as yet another way to make money14.
Mechanisms of action
DCA's supposed mechanisms of action against cancer cells relate to Warburg’s observations in the late 1920s that the metabolism of cancer cells is different from that of most normal cells15,16.
Shifting the metabolism from glucose oxidation within the mitochondria towards aerobic glycolysis in the cytosol might confer several survival advantages for the malignant cell: adaptation to a hypoxic microenvironment13, resistance to apoptosis17, and facilitated uptake of nutrients needed to proliferate16.It seems that to establish this altered metabolic phenotype multiple changes and molecular mechanisms around the signalling cascade of the hypoxia-inducible factors (HIFs) that mediate the effects of hypoxia on the cellular level are necessary8.
DCA inhibits pyruvate dehydrogenasekinase (PDK), which, in turn, inhibits the mitochondrial pyruvate dehydrogenase complex (PDC) by reversible phosphorylation. PDC catalyses the mitochondrial oxidative decarboxylation of pyruvate to acetyl–coenzyme A, allowing its entry into the Krebs cycle and away from lactate production18. Therefore, by inhibiting PDK, DCA maintains PDC in its active form, facilitating the mitochondrial oxidation of glucose. Chronic administration of the drug also may help stabilize PDC and decrease its rate of degradation8.
Bonnet et al. found that the metabolic shift from glycolysis to glucose oxidation triggered by DCA in cancer cells subsequently led to a number of pro-apoptotic changes of the mitochondria: decreased membrane potential, increased reactive oxygen species, and activated specific potassium channels1. By decreasing the expression of HIF-1 through a yet undetermined mechanism, DCA confers further apoptotic signals8. Kankotia and Stacpoole hypothesized that the decreased generation and release of lactic acid through DCA may alter the tumour-microenvironment and improve functions of immune cells. They further reported synergistic effects in vitro when DCA is combined with different drugs (e.g. bortezomib and arsenic trioxide) or radiation on a various cancer cell lines and supposed that DCA may not only promote apoptosis but also reverse resistance to chemotherapy and radiation8.
Prevalence of use
No data exists regarding the prevalence of use of DCA in cancer patients.
Legal issues
DCA is not legally available as a drug or supplement in the USA or in Europe other than through clinical trials, but is listed as an orphan drug19.
There has been an application for a patent for the use of DCA for the treatment of cancer which was subsequently withdrawn13. The FDA banned the purveyors from producing and selling DCA in the USA on July 17, 2007.
Controlled clinical trials
No controlled clinical trials of DCA in cancer patients are available.
Uncontrolled clinical trials
Please see table 1 for details of uncontrolled clinical trials of DCA for cancer.
Michelakis et al. conducted a phase I trial to evaluate the influence of DCA on glioblastoma (GBM) in five patients11. Three patients received oral DCA in increasing doses between 6.25 mg/kg and 25 mg/kg body weight twice daily as a sole treatment and two in addition to radiotherapy and temozolomide. The authors reported a partial response in one out of three patients that solely received DCA. Based on the results of this trial, the evidence that DCA is efficacious against GBM is very weak.
Garon et al conducted a phase I trial to evaluate the efficacy and safety of DCA in the treatment of advanced solid tumors. A total of seven patients, one with metastatic breast cancer and six with metastatic non-small cell lung cancer (NSCLC) received oral DCA at 6.25mg/ kg body weight twice daily. The best objective response until termination of the study was a stable disease in one patient after eight weeks. The results of this study gave no indication for a clinically relevant effect of DCA against breast cancer and NSCLC20.
Chu et al. performed a phase I clinical trial to evaluate the safety of DCA therapy and document possible tumor responses in 24 patients with advanced solid tumors refractory to treatment. Sixteen patients received oral DCA at 6.25mg/kg body weight twice daily for 28 days. In seven patients, DCA dose was escalated to 12,5mg / kg twice daily. Treatment was continued to a maximum of 28 days, until disease progression or unacceptable toxicity occurred. The best tumor responses reported were stable diseases in eight patients. Based on this phase I trial an effect of DCA in the treatment of advanced and treatment refractory solid tumors can neither be supported nor negated21.
Dunbar et al was a phase I trial to evaluate safety and tumor response to DCA treatment in 13 patients with high grade glioma and two patients with brain metastasis from adenocarcinoma of the uterus and the lung. Patient received oral DCA at 8 mg/kg body weight twice daily for 4 weeks. Dosing of DCA dosing was adapted depending on the genotype of glutathione transferase zeta 1 (GSTZ1). The best objective tumor responses after four weeks of treatment were stable diseases in eight patients. Based on these results, a limited effect of DCA on glioblastoma or brain metastases could be hypothesized22.
Case reports
Strum et al. contacted patients who reported responses of their cancers to DCA in internet forums and where possible assessed their medical records. One of the included patients was judged as being documented well enough to attribute a four year complete remission of a non-Hodgkin lymphoma relapse to the application of DCA23.
Another author reported two cases and attributed a long term complete remission after relapse from stage IV follicular lymphoma and a partial remission of chemotherapy resistant medullary thyroid carcinoma to DCA in combination with thiamine24,25.
In one case report DCA seemed to reduce swelling and pain from a metastasis of a poorly differentiated carcinoma26.
Adverse effects
There are several studies that reported a low toxicity profile of DCA when used over a short period of time5,27-30. However, animal studies did not provide a so-called “No Observed Adverse Effect Level” and found toxic effects of DCA on thekidney, peripheral nerves and the bone marrow1,4,6,31,32.
Peripheral neuropathies are the most frequently reported adverse effects in clinical trials with numbness, paresthesia and gait disturbance. Kaufmann et al. reported axonal sensory-motor peripheral neuropathy without demyelisation in adolescents and adults with a genetic mitochondrial disease who received 25 mg/kg/day DCA for several weeks or months33. In their phase I trial, Michelakis et al. reported no events of peripheral neuropathies at the 6.25 mg/kg dose level5. Although severe courses of neuropathies are reported34, most of the neurologic toxicities are regarded as being reversible; however, their regression can take up to several months35.
Other adverse effects that were reported in the phase I clinical trials in cancer patients were fatigue, gastrointestinal symptoms, and reversible hepatocellular injury9,36,37,23. One study with DCAwas closed prematurely due to safety concerns after the early death of two patients but it was unclear whether the early death was associated with the application of DCA20.
Contraindications
The following conditions might be regarded as contraindication for the use of DCA: history of allergic reactions attributed to halogenated organic acids, presence of intermediate or higher grade peripheral neuropathy due to co-morbidities (such as multiple sclerosis) and hepatic diseases.
Precautions/warnings
There are no data on the use of DCA during pregnancy and lactation. The majority of in vitro analyses did not show any mutagenic effects of DCA. However, in rats DCA has an embryotoxic and teratogenic effect with dose-dependent damages to the cardiovascular and uro-genital tract1. Several institutions, including the FDA have listed DCA as a possible “cancer-inducing” agent in humans.
Stacpoole reported mild sedative and/or anxiolytic effects after oral and parenteral administration of the DCA in occasional subjects37.
Interactions
As neurological toxicities often occur in patients being treated with chemotherapy, the risk of neuropathy could escalate3. This could, for instance, be the case for agents such as bortezomib, oxaliplatin, cisplatin, or thalidomide.
Results of cell culture studies suggest that DCA might reduce the cytotoxicity of cisplatin and doxorubicin but not that of temozolomide36.
Quality issues
DCA is often sold as industrial or technical grade, which might not be produced with the quality standards as pharmaceutical grade. Regarding the shelf life and storage the providers state that DCA can be stored for up to 1 year.
- BG Chemie. Toxikologische Bewertung: Dichloressigsäure, Natriumdichloracetat. Toxikologische Bewertungen - Ausgabe 03/06 Nr. 188b, 1-138. 2006.
- Ammini, C. V. & Stacpoole, P. W. Biotransformation, Toxicology and Pharmacogenomics of Dichloroacetate. In : Gribble, G. W. (ed.), Naturally Occurring Organohalgoen Compounds – a Comprehensive Update. Berlin: Springer Verlag, 2003, pp. 215-34.
- Michelakis, E. D., Webster, L. & Mackey, J. R. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 2008;99:989-94.
- Stacpoole, P. W., Nagaraja, N. V. & Hutson, A. D. Efficacy of dichloroacetate as a lactate-lowering drug. J Clin Pharmacol 2003;43:683-91.
- Chinnery, P., Majamaa, K., Turnbull, D. & Thornburn, D. Treatment for mitochondrial disorders. Cochrane Database Syst Rev 2006;1:CD004426.
- Stacpoole, P. W., Henderson, G. N., Yan, Z., Cornett, R. & James, M. O. Pharmacokinetics, metabolism and toxicology of dichloroacetate. Drug Metab Rev 1998;30:499-539.
- Agbenyega T, et al. Population kinetics, efficacy, and sadfety of dichloroacetate for lactic acidosis due to severe malaria in children. J Clin Pharmacol 2003;43:386-96.
- Kankotia, S. and P. W. Stacpoole. Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta 2014;1846:617-29.
- Shroads, A. L. et al. Age-dependent kinetics and metabolism of dichloroacetate: possible relevance to toxicity. J Pharmacol Exp Ther 2008;324:1163-71.
- Shroads, A. L., T. Langaee, B. S. Coats, T. L. Kurtz, J. R. Bullock, D. Weithorn, Y. Gong, D. A. Wagner, D. A. Ostrov, J. A. Johnson and P. W. Stacpoole . Human polymorphisms in the glutathione transferase zeta 1/maleylacetoacetate isomerase gene influence the toxicokinetics of dichloroacetate. J Clin Pharmacol 2012;52:837-49.
- Michelakis, E. D. et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2010;2:1-7.
- James, MO & Stacpoole, PW. Pharmacogenetic considerations with dichloroacetate dosing. Pharmacogenomics 2016;17:743-53.
- Gorsky D. The latest chapter in the seemingly never-ending saga of dichloroacetate as a cancer treatment (accessed on May 30, 2016)
- Geddes, L. Do-it-yourself Chemotherapy Access. Cancer World November/December, 2007;38-42.
- Warburg, O., Posener, K. & Negelein, E. Über den Stoffwechsel der Carcinomzelle. Biochem Ztschr 1924;152:309-44.
- Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029-33.
- Kim, J. W. & Dang, C. V. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res 2006;66:8927-30.
- Mathupala, S. P., Ko, Y. H. & Pedersen, P. L. Hexokinase-2 bound to mitochondria: cancer's stygian link to the "Warburg Effect" and a pivotal target for effective therapy. Semin Cancer Biol 2009;19:17-24.
- U.S. Food and Drug Administration. Search Orphan Drug Designations and Approvals [accessed on May 30, 2016].
- Garon, E. B., H. R. Christofk, W. Hosmer, C. D. Britten, A. Bahng, M. J. Crabtree, C. S. Hong, N. Kamranpour, S. Pitts, F. Kabbinavar, C. Patel, E. von Euw, A. Black, E. D. Michelakis, S. M. Dubinett and D. J. Slamon. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol 2014;140:443-52.
- Chu, Q. S., R. Sangha, J. Spratlin, J. V. L, J. R. Mackey, A. J. McEwan, P. Venner and E. D. Michelakis. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors." Invest New Drugs 2015;33:603-10.
- Dunbar, E. M., B. S. Coats, A. L. Shroads, T. Langaee, A. Lew, J. R. Forder, J. J. Shuster, D. A. Wagner and P. W. Stacpoole. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs 2014;32:452-64.
- Strum, S. B., O. Adalsteinsson, R. R. Black, D. Segal, N. L. Peress and J. Waldenfels. "Case report: Sodium dichloroacetate (DCA) inhibition of the "Warburg Effect" in a human cancer patient: complete response in non-Hodgkin's lymphoma after disease progression with rituximab-CHOP." J Bioenerg Biomembr 2013;45:307-15.
- Flavin, D. Medullary thyroid carcinoma relapse reversed with dichloroacetate: A case report. Oncol Lett 2010;1:889-91.
- Flavin, D. F. Non-Hodgkin's Lymphoma Reversal with Dichloroacetate. Volume 2010, Article ID 414726, 4 pages.
- Khan A. Use of oral dichloroacetate for palliation of leg pain arising from metastatic poorly differentiated carcinoma: a case report. J Palliat Med 2011;14:973-7.
- Calvert, L. D. et al. Dichloroacetate enhances performance and reduces blood lactate during maximal cycle exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2008;177:1090-4.
- Stacpoole, P. W. et al. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics 2006;117:1519-31.
- Fox, A. W. et al. Reduction of serum lactate by sodium dichloroacetate, and human pharmacokinetic-pharmacodynamic relationships. J Pharmacol Exp Ther 1996;279:686-93.
- Krishna, S. et al. Pharmacokinetics and pharmacodynamics of dichloroacetate in children with lactic acidosis due to severe malaria. QJM 1995;88:341-9.
- Theodoratos, A. et al. Phenylalanine-induced leucopenia in genetic and dichloroacetic acid generated deficiency of glutathione transferase Zeta. Biochem Pharmacol 2009;77:1358-63.
- Stacpoole, P. W., Kurtz, T. L., Han, Z. & Langaee, T. Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev 2008;60:1478-87.
- Kaufmann, P. et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology 2006;66:324-30.
- Brandsma, D., Dorlo, T. P., Haanen, J. H., Beijnen, J. H. & Boogerd, W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. J Neurol 2010;257:2099-100.
- Felitsyn, N., Stacpoole, P. W. & Notterpek, L. Dichloroacetate causes reversible demyelination in vitro: potential mechanism for its neuropathic effect. J Neurochem 2007;100:429-36.
- Heshe, D. et al. Dichloroacetate metabolically targeted therapy defeats cytotoxicity of standard anticancer drugs.
Cancer Chemother Pharmacol 2011;67:647-55. - Stacpoole PW: The pharmacology of dichloroacetate. Metabolism 1989;38:1124-44.
Citation
Hoeres T, Horneber M, CAM Cancer Collaboration. Dichloroacetate [online document], May 31, 2019.
Document history
Latest update: May 2019
Next update due: May 2022
Photo: Mostphotos.com